Abstract
Introduction The prognosis of ITP is age-related. In 80% of cases, children have a self-limiting course, whereas adults recover less frequently or relapse and overall ITP has a chronic course in ~70% of the cases. The risk of chronicity increases above the age of 10 years in pediatric cohorts, but data describing adolescents and young adults (AYAS) are lacking. Based on the assumption that adults are at greater risk of bleeding, treatment protocols and practice guidelines usually advise medical intervention. This is surely the right strategy for elderly patients or those with co-morbidities with indisputable higher bleeding risks. We assume that defining ITP in only two age categories might be an inaccurate oversimplification. AYAS have other needs, such as body appearance, mental health, social and financial issues. In addition, limitation of activity, side effects of steroids and risk of chronic disease are major reasons to adapt medical care. Even if AYAS have a similar risk of chronicity like adults, they may have greater potential to restore immune tolerance -in analogy to children- but with the help of an appropriate immunomodulatory therapy. The analysis of clinical data is the first step to design new strategies.
Methods Data were extracted from the PARC-ITP and the CARMEN-France registry, open since 2004 and 2013, respectively. PARC is an international multi-center registry collecting data prospectively of children and adults with newly diagnosed ITP. The CARMEN-France registry enrolls all incident ITP in adults 18 years from the French Midi-Pyrénées region, and since 2016 increasingly in other French centers. Demographics, diagnostic methods, clinical data, management are continuously documented in CARMEN and at defined timepoints in PARC (initial, at 6,12 months follow-up (FU), and then yearly). Patients 12-25 years old with initial platelet counts <100x10 9/l were included. Patients with secondary or misdiagnosed ITP (n=57), and pregnant women were excluded (n=10). Sustained remission was defined as a platelet count 100x10 9/l at 12 months (measured at 11-18 months) and without treatment for at least 6 months. For patients with borderline values (100-149 x10 9/l), later FUs were analyzed to determine the persistency of remission, and, if necessary, reassign patient's remission state. Only bleeding location, but not grade was analyzed in common. Data were analyzed with descriptive statistics.
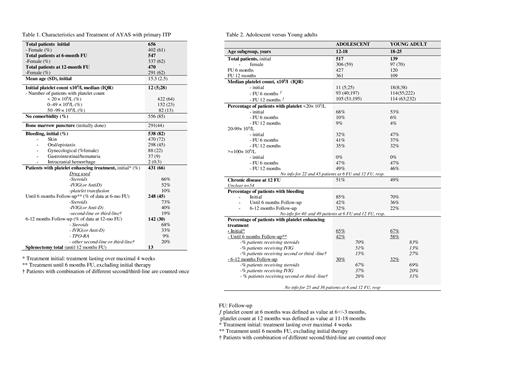
Results A total of 656 AYAS (61% female) with the initial diagnosis of primary ITP were recorded in the combined database until 2021. FU information was available for 547 (83%) and 470 (72%) patients at 6 and 12 months, respectively. Initial median platelet count was 12 x10 9/l (IQR 5). In 109 patients the diagnosis was incidental (17%), 538 patients suffered of bleeding symptoms (82%) (Table 1). At 6 months 49% of patients had platelets <100 x10 9/l. At 12 months 50% fulfilled the criteria of chronic disease, with a median platelet count of 57x10 9/l (IQR 32). Asymptomatic chronic ITP was reported in 40% (no bleeding between 6-12 months).
Platelet-enhancing drugs were reported in 66%, 45% and 30% at diagnosis, until 6 months and between 6-12 months, resp. Corticosteroids were preferred at all time-points, second-line treatments were various and given to 29% of patients with treatment beside 6 months (Table1).
There were no differences in initial diagnostic procedures, comorbidity, bleeding symptoms, median platelet counts, percentage of severe thrombocytopenia (<20 x10 9/l) and need of treatment and drug choice between women and men (besides gynecological bleeding). There were small differences in the subgroup of adolescents (12-18 years, 59% female) compared to young adults (18-25 years, 70% female), the later had more moderate thrombocytopenia at diagnosis, less bleeding at all FUs, but similar or even more treatments (Table 2).
Conclusion This is the first prospective project describing AYAS with a diagnosis of primary ITP. Analysis exhibited a clinical pattern among children and adults, with a risk of chronicity of 50%, and prolonged need of treatment (6-12 months) in 30% of cases. At diagnosis 17% had no bleeding signs, compared to 9% of children and 31% of adults in previous analysis of the PARC. There were no gender differences. Surprisingly, young adults experienced a greater number of non-bleeding phenotype than adolescents at all FUs, with comparable need of treatment between 6-12 months FU.
Schifferli: Novartis: Honoraria, Research Funding; Sobi: Honoraria. Moulis: Argenx: Membership on an entity's Board of Directors or advisory committees; Sobi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Godeau: Novartis: Consultancy; Grifols: Consultancy; Sobi: Consultancy; Amgen: Consultancy. Michel: Rigel: Honoraria; Argenx: Honoraria; UCB: Honoraria; Alexion: Honoraria; Amgen: Consultancy; Novartis: Consultancy. Grainger: Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Kuehne: Amgen: Research Funding; SOBI: Honoraria; UCB: Honoraria; Novartis: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal